rev2023.4.5.43379. Unlike charges exert attractive forces on each other and like charges exert repelling forces. q q Perrin showed that cathode rays actually carried negative electrical charge. Because opposite charges attract each other, the negative charge is attracted to the glass rod, leaving an excess positive charge on the opposite side of the right sphere. Virtual particle, Improving the copy in the close modal and post notices - 2023 edition. 10 Let us know if you have suggestions to improve this article (requires login).
You are really asking why like repels like and opposites attract at the smallest level. [AL]Ask students to define separation of charge. Electric charges move easily in an insulator but not in a conducting material. and you must attribute Texas Education Agency (TEA). is "Deep Down Things" By Bruce Schumm. Suspend it in a stand with the help of a thread. A proton with another proton would repel because they have the same charge. Every charged object sets up an electric field in the surrounding space. Coulomb's law, or Coulomb's inverse-square law, is a law of physics that describes force interacting between static electrically charged particles. When negative charge is transferred from one object to another, an excess of positive charge is left behind. Use the law of conservation of charge to find the final charge on the red sphere.
, so we can solve for Rubbing two surfaces together increases the transfer of electrons, because it creates a closer contact between the materials.
This virtual transfer of protons from a positive charge to a negative charge causes an attractive force between them. Point out that this static buildup is dissipated faster on humid days than on dry days. Can two like charges attract? The vast majority of positive charge in nature is carried by protons, while the vast majority of negative charge is carried by electrons. Yes, but this is really due to a different force, the Suppose that Millikan observed an oil drop carrying three fundamental units of charge. This process continued through your whole body until a distribution of excess electrons covered the extremities of your body. This is a measurement. The source of electrical charge is the attraction between protons and electrons in the atom, and the repulsion of each for its own kind. 1.602 No one can prevent nor prohibit thinking about these or other models. $$\vec{F}=\frac{kq^2}{r^2}\hat{r}$$ The net final charge of the system is
There are two types of charges which we see in nature: They are positive charges and negative charges.Like charges refer to positive-positive/negative-negative charges whereas unlike charges refer to positive-negative/negative-positive charges. If your reasoning is sound and your data are reliable, the conclusion demanded by the data must be seriously considered, even if that conclusion disagrees with the commonly accepted truth. It tells us that the net charge in a system is the same before and after any interaction within the system. Therefore $$\mathscr{L}=-mc^2\sqrt{1-\left(\frac{\dot{x}}{c}\right)^2} -\frac{q}{c} \left(cA_0 + A_m\dot{x}^m\right)$$
Positive and negative charges attract each other, Show more than 6 labels for the same point using QGIS. Charges are created when an atom gains an electron or loses an electron. When it gains an electron it becomes -ve charged and when it loses it beco q q q For physicist in universitary education this seems sometimes to be strange. WebWe observe in nature that electric charge can come in varying quantities, both positive and negative; and we observe that like charges repel, whereas opposite charges attract. Ask which type of force is at work between the balloon and the glass rod or comb (a repulsive force).
$$\int\left(-mc^2\sqrt{1-\left(\frac{\dot{x}}{c}\right)^2} -\frac{q}{c} \left(cA_0 + A_m\dot{x}^m\right)\right)dt$$ Can you travel around the world by ferries with a car? How do you tell if charges repel or attract? If like charges repel, why doesn't a charge break itself apart? Learn more about Stack Overflow the company, and our products. The answer is that no electrons actually traveled from your shoes to your hands.
comments sorted by Best Top New Controversial Q&A Add a Comment More posts you may like. Just before his death in 1981, Fletcher divulged that Millikan coerced him to give Millikan sole credit for the work, in exchange for which Millikan promoted Fletchers career at Bell Labs.
When you then touch a doorknob, some of your excess of electrons transfer to the neutral doorknob, creating a small spark. Sleeping on the Sweden-Finland ferry; how rowdy does it get?
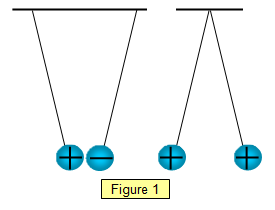
Thus, two positive charges repel each other, as do two negative charges. comments sorted by Best Top New Controversial Q&A Add a Comment More posts you may like. =1.602 I am not being glib or evasive in any way, but that's as near to. What happens if an excess negative charge is placed on a conducting object? 
Charge is a basic property of matter. The last is called deductive reasoning. WebElectrons and protons have equal charges which is why the atom as a whole is neutral. The same number of electrons is required to make 1.00 C of electric charge. Also point out that semiconductors are often made to act as insulators or as conductors, but not as materials with a conductivity that is between that of insulators and conductors. To make a handle, double over about 0.5 cm at one end so that the sticky side sticks together. Webwell the obvious answer is because such repulsive and attracting mechanism is built into the relationship of charged particles and its electric and magnetic field. The electron has a charge of the same magnitude but opposite signi.e., 1.602 1019 coulomb. r/explainlikeimfive ELI5 - Why do spacecraft/rovers always seem to last longer than they were expected to (e.g.
The "how" is given by the corresponding mathematical theory of quantum electrodynamics. If the potential is the same at two places (i.e., if the places have the same voltage), charges will not be influenced to move from one place to the other. Plastic object of small dimensions, such as comb or plastic stirrer. Similarly, two silk cloths rubbed in this manner will repel each other, because both cloths have negative charge. But why I go down and not up? They designed what is now a classic experiment performed by students. Rutherford found that most of the space occupied by the gold atoms was actually empty and that almost all of the matter of each atom was concentrated into a tiny, extremely dense nucleus, as shown by the right-side image of Figure 18.3. The attraction or repulsion acts along the line between the two charges. 6 
Slowly peel off the two pieces by pulling on the handle of the bottom piece. Figure 18.7 shows two spheres that initially have +4 C and +8 C of charge. Every constituent of matter has an electric charge with a value that can be positive, negative, or zero.
The law of conservation of charge says that electrical charge cannot be created or destroyed. 10 Charges that are opposite (positive and negative) draw one another together or attract. Discuss how moving electrons to the right is equivalent to moving the same magnitude of positive charge to the left, but be sure to clarify that, in most situations, only negative charges actually move in solids. After the two spheres interact, the blue sphere has a charge of +10 C. The law of conservation of charge allows us to find the final charge, Materials can be arranged according to their ability to conduct electric charge. What is the difference between a physical theory and mathematical theory? ) Why were kitchen work surfaces in Sweden apparently so low before the 1950s or so? This means that the existence of opposite charges assigned to particles is a given of nature, a law. Later, Thomsons work led him to declare, I can see no escape from the conclusion that [cathode rays] are charges of negative electricity carried by particles of matter..
10 In Coulombs law, however, the magnitude and sign of the electric force are determined by the electric charge, rather than the mass, of an object. Figure 18.2 shows how these simple materials can be used to explore the nature of the force between charges. final Omissions?
[AL]Ask whether students recall other conductors and insulators in physics.
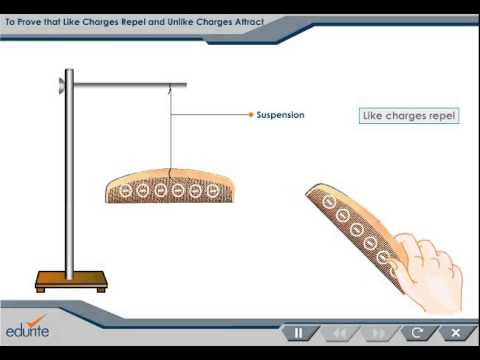 It's a fundamental force in the Universe. For example, the distribution of charges in conductors is generally unknown because the charges move freely within the conductor.
It's a fundamental force in the Universe. For example, the distribution of charges in conductors is generally unknown because the charges move freely within the conductor.
The force and conservation laws are only two aspects of electromagnetism, however.
It may not look gauge invariant at first, but after adding gauges, one sees it is indeed gauge invariant. is the net charge of the system before the interaction, and  red When a positive charge is brought closer to a negative charge, excess number photons from the positive charge get transferred to the negative charge, so that the number of photons in both the charges are balanced. Like charges repel each other; unlike charges attract. People know that it is true, because it has been observed to be true. Also Read: Which Has A Higher Gravitational Pull, A Stationary Or A Rotating Object?
red When a positive charge is brought closer to a negative charge, excess number photons from the positive charge get transferred to the negative charge, so that the number of photons in both the charges are balanced. Like charges repel each other; unlike charges attract. People know that it is true, because it has been observed to be true. Also Read: Which Has A Higher Gravitational Pull, A Stationary Or A Rotating Object?
q The upper comb has excess electrons, and the excess electrons in the rubber belt get transferred to the comb by conduction. This balanced nature of the atoms is the reason why atoms are existing. A positive charge contains an excess number of protons in it whereas a negative charge contains a less number of protons in it.  (b) A positively charged rod approaches, which attracts negative charges, leaving excess positive charge on the right sphere. 19 To subscribe to this RSS feed, copy and paste this URL into your RSS reader. 10 inkdrop What would be the net charge on this oil drop? How do we know that charges interact by photons?
(b) A positively charged rod approaches, which attracts negative charges, leaving excess positive charge on the right sphere. 19 To subscribe to this RSS feed, copy and paste this URL into your RSS reader. 10 inkdrop What would be the net charge on this oil drop? How do we know that charges interact by photons? 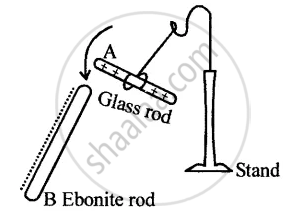 Note that this satisfies Newton's third law because it implies that exactly the same magnitude of force acts on q 2.Coulomb's law is / This saying is based on electric charge, which is a property of matter that causes objects to attract or repel each other.
Note that this satisfies Newton's third law because it implies that exactly the same magnitude of force acts on q 2.Coulomb's law is / This saying is based on electric charge, which is a property of matter that causes objects to attract or repel each other.